
*This has to do with something called a mass defect, which occurs because of the nuclear binding effect-negative potential energy associated with attractive forces holding the nucleus of the atom together. One other reason is that the atomic masses of the two isotopes are both less than the numbers in their names: has a mass of 64.9278 amu, and has a mass of 62.9396 amu*, so plugging these into the formula above would also contribute to the atomic mass of Cu being a value smaller than 64. The metal has a density of 8.96 Mg/m3 and a melting.

(percent abundance of first isotope)*(atomic mass of first isotope)+(percent abundance of second isotope)*(atomic mass of second isotope)īased on the mass given in the periodic table, the relative abundance of Copper-63 is larger than that of Copper-65 because the atomic mass of Cu is closer to 63. Copper is a metal in group IB of the periodic table with the atomic number 29 and an atomic weight of 63.54. The atomic mass of an element on the periodic table is given by:

How do we find atomic mass For any given isotope, the sum of the numbers of protons and neutrons in the nucleus is called the mass number.
#Atomic mass of copper code
The genetic code is a set of three-nucleotide sets called codons and each three-nucleotide combination designates an amino acid for example AUG. Where more than one isotope exists the value given is the abundance weighted average. So 1- g of Copper contains 6.022/63.54 x 10²³ 9. The atomic mass of most copper atoms is about 63 amu because of their 29 protons and 34 neutrons.
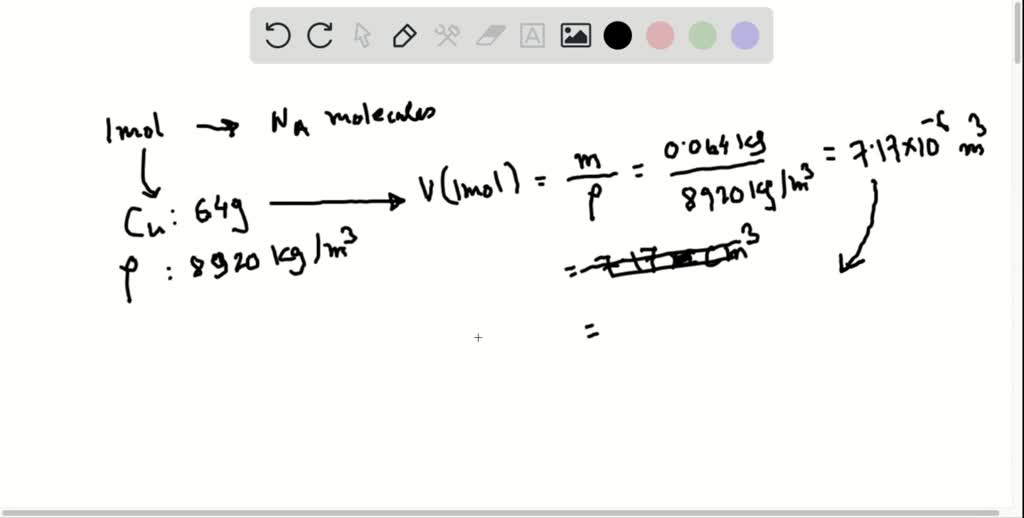
What is the atomic number of 19 Potassium is a chemical element with symbol K and atomic number 19. Atomic weight of Copper 63.54- g So 63.54- g of Copper contains 6.022x10²³ - atoms. This has to do with the relative abundances (abundance=what percentage of all the element in existence is that particular isotope) of the two isotopes. For April, we have selected copper, a transition metal with chemical symbol Cu and atomic number 29. Copper has high density, melting point and boiling point.


 0 kommentar(er)
0 kommentar(er)
